medical package stability test|medical device stability testing : broker Assurance that your package will provide an effective, consistent sterile barrier for your medical device requires a well-designed, thoroughly documented test protocol evaluating both seal . web21 de mar. de 2022 · 2 min de leitura. Imagem: Paramount/Reprodução. Luciana Penante. via nexperts. Após o sucesso de Pânico 5, lançado no início de 2022 após um hiato de .
{plog:ftitle_list}
webfoquinha @fooquinha - Último acesso: há 2 dias. 24054 Followers View Profile .
Product and Package Stability Studies: The Application of FDA Guidance The United States Food and Drug Administration provides guidance on physi-cal tests to employ to demon-strate that packaging main-tains the sterility of products throughout their shelf life. This article .
auto gas analyzer
Assurance that your package will provide an effective, consistent sterile barrier for your medical device requires a well-designed, thoroughly documented test protocol evaluating both seal . A package stability evaluation demonstrates the sterile barrier system maintains integrity over time. So when we say the term “package stability/expiration,” we’re referring to the date the product should be used. . in this case medical device pouches (F88 1.1). This test method measures the force required to separate one side of a fin . Minimally, the package test methods must include one of the ISO Package Tests for Seal Strength and one of the ISO Package Tests for SBS Integrity; in this example, microbial barrier by both closure integrity and .
Package (aka Container-closure): Primary package components. In direct product contact (or may be) Secondary package components critical for ensuring package assembly . E.g., aluminum crimp seal on vial/stopper . Product-Package: The primary package with critical secondary components (the container-closure system) AND . The packaged contents .ASTM F1980: Standard for Accelerated Aging of Sterile Barrier Systems and Medical Devices. ASTM F1980, also a test standard titled, “Standard Guide for Accelerated Aging of Sterile Barrier Systems and Medical Devices” is a testing procedure that is used to help with the assessment of the sterile integrity of a package and product designed for medical use.Other Test Methods Related to ASTM F1980. For additional test methods related to ASTM F1980 we invite you to read on Accelerated aging and ASTM D3045. If you have any questions about the ASTM F1980 test or other material tests, we invite you to contact our material testing laboratory. We will be happy to answer your questions and help you with .
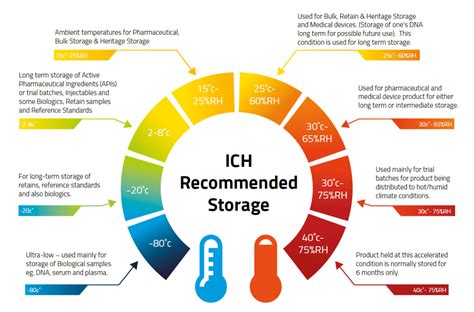
309 Annex 10 Stability testing of active pharmaceutical ingredients and finished pharmaceutical products Introduction and background The guidance on Stability testing of active pharmaceutical ingredients and finished pharmaceutical products was published as Annex 2 in the World Health Organization (WHO) Technical Report Series, No. 953, 2009 (1).The aim of these regulatory .
Product and Package Stability Studies The Application of FDA Guidance Introduction In February 2008, the FDA issued ‘Guidance to Industry’ dealing with physical tests . The same pack can then be perforated and used to challenge the test method. Medical device manufacturers often question the ‘hole’ size used for the validation of .
ICH Q1C Stability testing: requirements for new dosage forms; ICH Q1D Bracketing and matrixing designs for stability testing of drug substances and drug products - Scientific guideline; ICH Q1E Evaluation of stability data; ICH Q1F Stability data package for registration in climatic zones III and IV
Shelf Life Stability Testing (accelerated aging and real-time aging) . These include reduced damage, lower returns, and improved customer satisfaction and reputation. Test standards that simulates the transportation cycle includes: ISTA Series 1; . If you require medical package testing and the need to validate healthcare packaging, you .Barrier properties: Medical device packaging must provide an effective barrier against external elements. Accelerated aging tests how well the packaging materials will be able to maintain sufficient barrier properties. Color stability: Light-sensitive components must maintain color stability within their packaging. Accelerated aging can predict .test reports that validate packaging stability using accelerated aging studies, pending receipt of data from real-time aging assessments. Accelerated aging studies are normally conducted in accordance with the standardized test methods described in ASTM F 1980, Standard Guide for Accelerated Aging of Sterile Medical Device Packages.
Test the stability and accurately measure the health of your broadband network connection; cable, dsl, fiber or wifi internet speed of all your devices online with bandwidth to locations around the world. Check how fast is your download and upload speeds in Mbps, and the quality and performance of your ISP with our top-notch speed test diagnostic tool.performance, stability testing for expiry dating should be conducted along with package stability testing . 6.4.6 If accelerated aging tests are performed, a documented rationale for the accelerated aging conditions and test duration chosen shall be established.
The creep test is based on internal pressurization, similar to the burst test listed above. This part of package testing maintains a specific pressure in the package for a set amount of time (or until the package fails). Creep testing is used for flexible packaging of differing sizes and seal configurations. ASTM F2096: Bubble emission testQ.I. Medical makes unique products for pharmacists and nurses who handle sterile solutions. Our focus is on devices, test kits & accessories that improve aseptic technique. Applications include environmental monitoring, technique . Stability Testing (accelerated and real-time aging): Stability Testing is a race against time but in fast forward. Here, labs simulate the effect of time on packaging using controlled conditions like elevated temperatures. .The purpose of stability testing is to provide evidence on how the quality of an active substance or finished product varies with time under the influence of a variety of environmental factors such as temperature, humidity, and light, and to establish a re -test period for the active substance or a .
Instead, filled packages are saturated with helium before they are sealed. An operator places a sample package in the test chamber, where a sensor measures any escaping helium. The test measures leaks at a rate as low as 1 x 10 –9 cm 3 /sec, compared to the vacuum test's lowest leak rate of 1 x 10 –4 cm 3 /sec. "The SIMS unit is designed to .
Satisfy requirements by ensuring the integrity of sterile medical device packaging within ISO 11607 compliance. Medical Device package validation incorporates package stability testing, performance/dynamics testing, and strength and integrity testing .
product and packaging stability studies
Comprehensive ASTM F1980 and ISO 11607 accelerated & real-time aging testing to demonstrate the shelf life of a package or product. Skip to content . ISO 11607 requires sterile medical device manufacturers to demonstrate the shelf life of their packaging system. . Real time aging is a stability testing process used to provide real data to . Real-time aging (stability) is the requirement of ANSI/AAMI/ISO 11607-1: 2019. 1.5 Methods used for sterile barrier system performance validation, which include, environmental challenge, distribution, handling, and shipping events, are used for package performance (event-related loss of integrity) testing and are beyond the scope of this guide.Preshipment Test Procedures o ISO 4180-1: Complete, filled transport packages – General rules for the compilation of performance test schedules – Part 1: General principles The documents are listed in ISO 11607-1, Annex B, as standardized test methods, that may be used to demonstrate compliance with the requirements of this standard 27
Jennifer Gygi is an Expert Technical Consultant at Nelson Laboratories; a microbiological testing company specializing in improving the quality of life by ensuring medical products are safe, sterile, and functional.Gygi has over 26 years of laboratory experience at Nelson Labs. She has worked in the Microbiology, Bioburden, Organism IDs, Packaging, and .Medical device package testing uses a series of test standards to evaluate product and package safety in manufacturing, transit, storage, and in-use environments. . Stability testing (via accelerated and real-time aging) must demonstrate that the sterile barrier system remains intact throughout the expected shelf-life of the product .DDL – MN DDL, Inc. 10200 Valley View Road Eden Prairie, MN 55344 Phone: 952-941-9226 Fax: 952-941-9318 For the current experiment, the accelerated aging test was performed in compliance with guidelines of the American Society for Testing and Materials (ASTM) F 1980-2 Standard Guide for Accelerated Aging of Sterile Medical Device Packages for the purposes of assessing the thermal stability over time of the Mint Lift ® 17 and the Mint Lift .
Packaging Compliance Labs (PCL) is an ISO 17025-accredited lab that offers medical device packaging validation, packaging engineering, and ISO 13485-certified contract packaging.
product and package stability testing
POP! Slots Vegas Casino Games é um jogo cassino desenvolvido pela PLAYSTUDIOS INC. O BlueStacks App Player é a melhor plataforma para jogar este jogo Android no seu PC ou Mac e com ele ter uma experiência Android imersiva. Traga a emoção de Las Vegas para qualquer lugar do mundo com o POP! Slots Vegas Casino Games.
medical package stability test|medical device stability testing